How a Centrifuge Works
|
by Ivan Oelrich and Ivanka Barzashka
Uranium powers both nuclear reactors and nuclear bombs. However, uranium cannot be used in its natural form-- it must be processed to increase the concentration of the active isotope of uranium, U-235. Most chemical elements have different isotopes, which are atoms with slightly different weights resulting from the different number of neutrons in the nucleus. While the chemical properties of two isotopes are almost identical, the difference in the number of neutrons in the nucleus can result in nuclear properties that are dramatically different. Such is the case with uranium, which is made up of two isotopes, the predominant uranium-238 (or U-238) and U-235, which is only 0.7% of natural uranium. It is the U-235 that can be directly split, or fissioned, to produce power in most of the world’s current commercial nuclear reactors. (Thus, materials like U-235 and plutonium are called “fissionable".) The U-238 is not directly fissionable. To be useful, the concentration of U-235 must be increased to 3-5 percent for a typical commercial reactor and to 80-95 percent for a nuclear weapon.
Because the two isotopes of uranium are chemically indistinguishable, physical methods that exploit the small difference in weight must be used to enrich uranium in the 235 isotope. Historically, several techniques have been used but the method that is overwhelming preferred today is a gas centrifuge.
Surprisingly, for such a heavy metal, uranium can form a compound that is a gas at moderate temperatures. Uranium combines with six atoms of fluorine to form uranium hexafluoride (UF6). At room temperature and pressure, UF6 is a white solid but it turns to a vapor or gas at moderate temperature (133˚ F or 56˚ C at normal atmospheric pressure).
Coincidentally, fluorine has only one stable isotope, F-19. This is important because, if the fluorine atoms had different weights, there would be no way to distinguish whether the difference in weight of the UF6 molecule was due to the uranium or the fluorine atoms. Since fluorine atoms are identical, any change in mass has to be due to the different uranium isotopes.
|
|
|
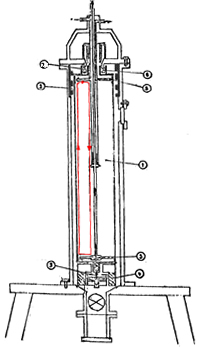
EG-1802 Soviet Centrifuge
|
The heart of a gas centrifuge is a tube, called the rotor, that spins at high speed around its long axis. The performance of the centrifuge depends critically on the speed of the rotor. Technical advances in materials, high-speed bearings, and precision machining are what have made increased rotor speeds possible and made centrifuges practical. Today, rotors may spin in excess of 60,000 rpm with the outside surface of the rotor moving well in excess of the speed of sound.
The spinning rotor creates powerful centrifugal forces that mimic a miniature gravitational field except, of course, “up” is toward the axis of the rotor and “down” is toward the outer rim. The Earth’s atmosphere is densest at the surface and, as altitude increases, each layer of air has less air above it pressing down on it and compressing it. Hence, the pressure and resulting density decrease as we go up through the atmosphere. A similar effect occurs in a centrifuge but, because the forces created by the fast spinning centrifuge might be a million times stronger than gravity, everything happens on a much smaller scale.
In the spinning rotor, the slightly heavier UF6 that contains U-238 will be slightly more compressed along the rim relative to the lighter UF6 that contains U-235, which would have a relatively greater concentration near the axis. The separation between the two isotopes created by the centrifugal forces is quite small. However, there is a simple way to substantially increase the degree of separation by creating a circulation flow along the length of the centrifuge. If one end of the centrifuge is heated, the warmer gas will rise at that end and flow toward the opposite end along the axis, while cooler gas will flow in along the wall to replace it. Alternately, a scoop used for removing gas at one end will slow the flow of gas, reducing the centrifugal force and allowing the gas to rise. Now the centrifuge has a flow of gas along the center toward one end and a flow along the wall toward the other end.
As in any gas, the molecules of UF6 are not static, rather individual molecules have thermal energy and are moving around within the volume of gas. To say that the concentration of U-238 is higher along the wall and U-235 higher along the center, simply means that, statistically, each molecule containing 238 will spend more time on average along the wall than a molecule containing U-235, which will, statistically, spend a relatively longer time near the center. Being more often near the wall, the U-238 will get pushed along with the wall flow in the direction of the hot or scoop end of the rotor more often than it will get pushed along the center flow, thereby increasing its concentration at that end. The U-235 will spend, on average, more time being pushed along with the flow along the center and will concentrate at the opposite end. Thus, the U-235 and U-238 do not separate relative to the wall and the center but along the length of the centrifuge. This is one reason that the longer the rotor, the greater the separation of the centrifuge. Tubes running along the center of the rotor extract the UF6 gas. From one end the gas will be slightly enriched in U-235 compared to the feed gas and at the opposite end the gas will be slightly depleted in U-235 compared to the feed gas.
One of the key determinants of a centrifuge’s performance is the speed of rotation and this depends on the material the rotor is made of. Because only a small amount of UF6 is in the centrifuge at any given moment, most of the force on the spinning centrifuge is due to the centrifuge material itself. The important characteristic of the centrifuge material is, therefore, not just its strength but the ratio of its strength and density. For example, two common materials used for centrifuge rotors are aluminum and steel. Aluminum is only about a quarter as strong as steel but that lesser strength is compensated by a density that is a third lower, so the much weaker, but lighter, aluminum is able to spin almost as fast as the stronger, but heavier, steel.
High spin rates require very precise balance of the rotor so another critical technology is high precision machining of the rotor components. High speed, low wear and low friction bearing are also essential. Finally, UF6 is highly corrosive so all parts that are exposed to the gas must be made of resistant materials.
 |
|
Urenco Centrifuge Cascade
|
No single centrifuge can achieve an adequate degree of enrichment and the amount of material that a single centrifuge can handle is small. To process sufficient material, centrifuges, sometimes hundreds, are operated in parallel. To get the necessary degree of enrichment, the enriched output of one set of centrifuges will be fed in as input to another set of centrifuges for further enrichment. Each set of centrifuges enriches the uranium a bit more than the previous until the desired enrichment is achieved. Such a collection of centrifuges is called a cascade and any given set of centrifuges operating in parallel is called a stage. The other part of the output of the first stage will, of course, be slightly depleted in U-235. It will, nonetheless, still contain significant quantities of the valuable isotope, so it is not simply thrown away. It is fed into centrifuges that enrich the depleted material back up to the original concentration and then fed back into the enriching cascade. The stages above the initial feed point that enrich the uranium above its original concentration are the enriching stages and those that operate below the original concentration are called stripping stages.
A cascade to supply a large nuclear reactor may require several thousand centrifuges of moderate performance. Depending on the enrichment of each stage and the desired degree of enrichment, a cascade may have anywhere from a few stages to more than twenty.
Centrifuges raise serious nuclear weapons proliferation concerns because exactly the same machines that are used to enrich uranium for a nuclear reactor can enrich uranium for a nuclear bomb. In general, a nuclear reactor needs a small degree of enrichment of a large amount of material and a bomb needs a large degree of enrichment of a small amount of material. Exactly the same centrifuges can do either job; the only change required is how they are piped together into a cascade. Moreover, a single typical large commercial nuclear power plant may have ten times more separative work than is needed to produce one uranium bomb per year, so even a modest commercial enrichment facility has a significant nuclear weapons production capability.